As food safety standards continue to improve, the EU has recently updated and adjusted its food regulations. This article will introduce the latest regulatory requirements that food companies exporting to the EU must be aware of.
1. Expanded scope of use of genetically modified rapeseed GT 73
Main regulatory contents:
The European Commission recently published Implementing Regulation (EU) 2024/388, which marks the expansion of the use of genetically modified rapeseed GT 73 in food. According to the new regulation, products containing, consisting of or produced from genetically modified rapeseed MON-73-7 are now allowed to be used in food, food ingredients and feed, except for planting.
Compliance Notes:
1. The product label and accompanying documents must clearly state "not for planting".
2. Exporting companies should ensure that their products comply with the requirements of Regulation EU 2024/388.
3. Companies should pay close attention to further notifications and guidance from EFSA to ensure full compliance.
2. The application scope of new foods of isomaltooligosaccharides is expanded
Main regulatory contents:
According to document No. 2024.8543 issued by EFSA, isomaltooligosaccharides are now intended to be used as a new type of food in specific populations, with an allowed intake of 30 grams per day and a total intake not exceeding 142 grams per day.
Compliance Notes:
1. Food supplement products should clearly label the recommended daily intake.
2. Companies should ensure that the nutritional content and labeling information of their products comply with the latest regulatory requirements.
3. During promotion and marketing, avoid misleading consumers about the product’s purpose and safe intake.
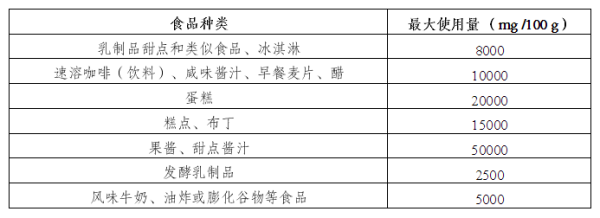
3. The scope of use of Quillaja extract as a food additive is expanded
Main regulatory contents:
The European Union plans to expand the use of Quillaja extract (E 999) as an additive in food. The specific requirements will be officially published in the Official Journal of the European Union.
Compliance Notes:
1. It is necessary to clarify the proportion of soap tree extract used in the product.
2. Make sure the correct additive number (E 999) is on the product label.
3. Comply with the usage limits of Quillaja saponaria extract in specific food categories.

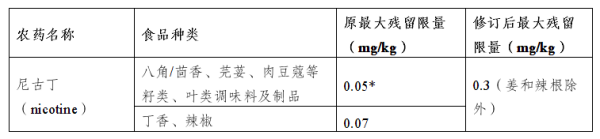
IV. Modification of the maximum residual amount of nicotine in certain products
Main regulatory contents:
Regulation (EU) 2024/451 amends the maximum residual levels of nicotine in certain products to ensure food safety.
Compliance Notes:
1. Food manufacturers should re-evaluate the nicotine content in their products.
2. Update testing methods and processes to ensure compliance with new residue limits.
3. Update product labels and specifications to ensure the accuracy and transparency of consumer information.
As EU food regulations continue to be updated, food companies exporting to the EU must remain highly vigilant and adjust their production, labeling and testing processes in a timely manner to ensure that their products fully comply with new regulatory requirements.


 Follow customer service WeChat
Follow customer service WeChat