1. Background
On December 23, 2023, the Turkish government officially announced the official release of the revised regulations on the Registration, Evaluation, Authorization and Restriction of Chemicals (KKDIK). This new regulation adjusts the official registration deadline for chemicals and manages them in stages based on the annual production or import volume and the hazard category of the substance. This revision is particularly important for companies operating in the Turkish market, especially those involved in large-scale chemical production or import.
II. Main contents of the new regulations
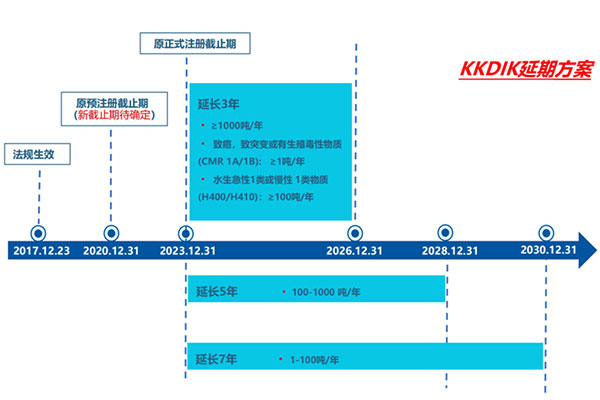
1. New deadline for formal registration
? Before December 31, 2026: Registration of substances produced or imported ≥1,000 tons per year needs to be completed; Aquatic Acute 1 and Aquatic Chronic 1 (H400, H410) hazard categories, substances produced or imported ≥100 tons per year; CMR 1A/1B hazard categories, substances produced or imported ≥1 ton per year.
? Before December 31, 2028: Complete registration for substances produced or imported in quantities of 100-1000 tons per year.
? Before December 31, 2030: Complete registration for substances produced or imported in quantities of 1-100 tons per year.
2. The pre-registration deadline is not determined
Currently, the new revised regulations do not specify the deadline for pre-registration. It is expected that the Ministry of Environment and Urbanization (MoEUCC) of Turkey will release relevant details through subsequent announcements.
3. Adjustment of LR (Lead Registrant) election process
The Chamber of Commerce and the Union of Commodity Exchanges will manage the LR election process to enhance the transparency and professionalism of the process and address data and cost-sharing issues.
4. Implementation and enforcement
The revised regulations will be officially implemented from the date of publication. The Turkish government will strengthen the supervision and enforcement of unregistered substances.
3. Enterprise response strategies
1. Actively prepare for registration
Even though the new timetable seems relaxed, given the current progress of Turkey’s joint registration and the status of data sharing in Europe, companies still need to actively prepare, especially those with high tonnage registration needs.
2. Pay attention to the announcement of the pre-registration deadline
Companies should pay close attention to the MoEUCC’s subsequent announcements regarding pre-registration deadlines to ensure that all necessary registration steps are completed in a timely manner.
3. Reasonable planning of resources
Rationally arrange resources and time according to the new phased registration requirements to ensure that the registration task is completed on time.
4. Strengthen compliance management
Strengthen internal compliance management to ensure that all chemicals sold or used in the Turkish market comply with the latest KKDIK regulations.
5. Data sharing and collaboration
Collaborate with other businesses to share data during the registration process, especially for multiple registrants of the same chemical, to reduce costs and improve efficiency.


 Follow customer service WeChat
Follow customer service WeChat